What is battery.
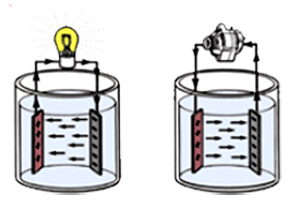
The battery stores electricity in the form of chemical energy. Through a chemical reaction process the battery creates and releases electricity as needed by the electrical system or devices.Since the battery loses its chemical energy in this process, the battery must be recharged by the alternator. By reversing electrical current flow through the battery the chemical process is reversed, thus charging the battery. The cycle of discharging and charging is repeated continuously and is called “battery cycling”

Deep Cycling
Although batteries do cycle continuously, they do not cycle deeply. Deep cycling is when the battery is completely discharged before recharge. Automotive batteries are not designed as deep cycle batteries. Automotive batteries are designed to be fully charged when starting the car; after starting the vehicle, the lost charge is replaced by the alternator. So the battery remains fully charged. Deep cycling an automotive battery will cause damage to the plates and shorten battery life. Deep Cycle Batteries on the other hand are designed to be completely discharged before recharging. Because charging causes excessive heat which can warp the plates, thicker and stronger plate grids are used. Normal automotive batteries are not designed for repeated deep cycling and use thinner plates.
The Automotive Battery
A lead-acid storage battery is an electrochemical device that produces voltage and delivers electrical current. The battery is the primary “source” of electrical energy used in vehicles today. It’s important to remember that a battery does not store electricity, but rather it stores a series of chemicals, and through a chemical process electricity is produced. Basically, two different types of lead in an acid mixture react to produce an electrical pressure called voltage. This electrochemical reaction changes chemical energy to electrical energy and is the basis for all automotive batteries. An automotive battery is a type of rechargeable battery that supplies electric energy to an automobile.Usually this refers to an SLI battery (starting, lighting, ignition) to power the starter motor, the lights, and the ignition system of a vehicle’s engine

DRY CHARGED:
The battery is built, charged, washed and dried, sealed, and shipped without electrolyte. It can be stored for up to 12 months. When put into use, electrolyte and charging are required. Batteries of this type have a long shelf life.
MAINTENANCE FREE:
The lead-acid battery is filled with electrolyte and charged when it is built. During storage, a slow chemical reaction will cause selfdischarge. Periodic charging is required. Most batteries sold today are Maintenance Free.
The Purpose of a Battery
The battery supplies electricity when the:
ENGINE IS OFF:
Electricity from the battery is used to operate lighting, accessories, or other electrical systems when the engine is not running.
ENGINE IS STARTING:
Electricity from the battery is used to operate the starter motor and to provide current for the ignition system during engine cranking. Starting the car is the battery’s most important function.
ENGINE IS RUNNING:
Electricity from the battery may be needed to supplement the charging system when the vehicle’s electrical load requirements exceed the charging system’s ability to produce electricity. Both the battery and the alternator supply electricity when demand is high.
Terms and ratings
• Ampere-hours (Ah) is a measure of electrical charge that a battery can deliver. This quantity is one indicator of the total amount of charge that a battery is able to store and deliver at its rated voltage. Its value is the product of the discharge-current (in amperes), multiplied by the duration (in hours) for which this dischargecurrent can be sustained by the battery. Generally, this value (or rating) varies widely with the duration of the discharge period (see: Peukert’s Law), therefore the value is typically only meaningful when the duration is specified. This rating is rarely stated for automotive batteries, except in Europe where it is required by law.
• Cranking amperes (CA), also sometimes referred to as marine cranking amperes (MCA), is the amount of current a battery can provide at 32°F (0°C). The rating is defined as the number of amperes a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12 volt battery).
• Cold cranking amperes (CCA) is the amount of current a battery can provide at 0°F (−18°C). The rating is defined as the current a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12-volt battery). It is a more demanding test than those at higher temperatures.
• Hot cranking amperes (HCA) is the amount of current a battery can provide at 80°F (26.7°C). The rating is defined as the current a lead-acid battery at that temperature can deliver for 30 seconds and maintain at least 1.2 volts per cell (7.2 volts for a 12-volt battery).
• Reserve capacity minutes (RCM), also referred to as reserve capacity (RC), is a battery’s ability to sustain a minimum stated electrical load; it is defined as the time (in minutes) that a lead-acid battery at 80°F (27°C) will continuously deliver 25 amperes before its voltage drops below 10.5 volts.
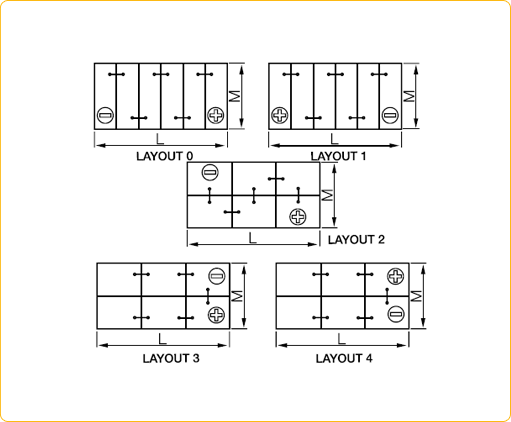
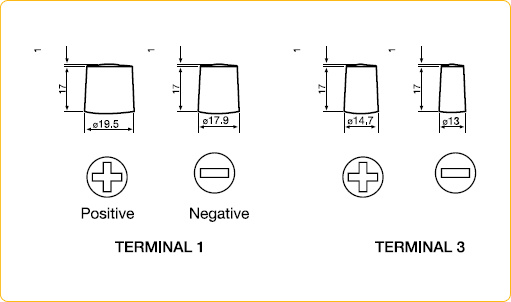
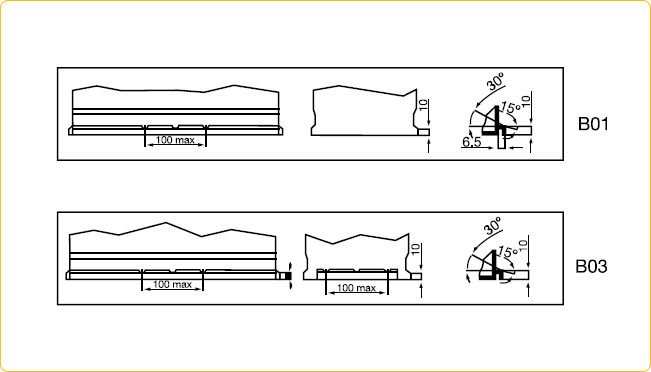
• Battery Council International group size (BCI) specifies a battery’s physical dimensions, such as length, width, and height. These groups are determined by the Battery Council International organization.
• Peukert’s Law expresses the fact that the capacity available from a battery varies according to how rapidly it is discharged. A battery discharged at high rate will give fewer ampere hours than one discharged more slowly.
• The hydrometer measures the density, and therefore indirectly the amount of sulfuric acid in the electrolyte. A low reading means that sulfate is bound to the battery plates and that the battery is discharged. Upon recharge of the battery, the sulfate returns to the electrolyte